CONCLUSIONS
Peripheral T-cell lymphomas (PTCLs) comprise a heterogeneous group of aggressive lymphoproliferative disorders with dismal clinical outcomes. In China, PTCLs account for over 20% of non-Hodgkin lymphoma (NHL) cases, representing a relatively high incidence. Although CHOP-like regimens remain the standard frontline therapy, their efficacy is limited (Alain Mina, 2022). Previous studies have demonstrated high expression of XPO1 in T-NHL cell lines and potent anti-proliferative effects of the selective inhibitor selinexor at nanomolar concentrations (Abeykoon JP, 2019). Here, we report the safety and preliminary efficacy of combining selinexor with CHOP as first-line treatment in previously untreated PTCL patients (ChiCTR2200057942).
In this open-label, multicentre, phase 2 trial, we enrolled patients with previously untreated peripheral T-cell lymphoma (PTCL). Participants received standard CHOP chemotherapy on day 1 of each cycle for 6 or 8 cycles, along with selinexor priming at 40 or 60 mg on days 1, 8 and 15. Patients achieving a complete response (CR) or partial response (PR) at the end of induction treatement (EOI), with or without autologous stem cell transplantation (ASCT), and then received maintenance treatment with selinexor (days 1, 8, 15 and 22 every 28 days) for up to 12 months. The primary endpoint was overall response rate (ORR) during induction, and secondary endpoints included CR rate, duration of response (DOR), PFS, OS and safety.
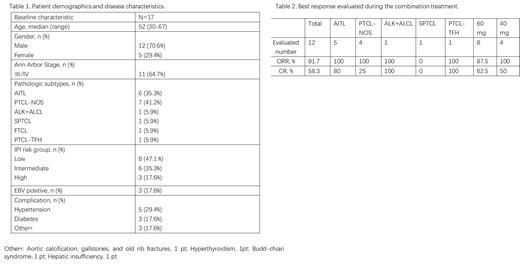
Between Jul 1, 2022 and May 21, 2023, a total of 17 previously untreated PTCL pts were enrolled and received treatment at six centers, including seven with AITL (41.2%), six with PTCL-NOS (35.3%). The median age was 52 years (range 30-67), 11 (64.7%) had stage III/IV disease, with nine (52.9%) pts having IPI 2-5. As of July 10, 2023, with a median follow-up of 7.3 months (range 1.9-14.8 months), three pts discontinued treatment without efficacy evaluation (two pts due to bone marrow suppression, one pt due to COVID-19 infection), and one pt died due to disease progression. Among 12 pts evaluable for the primary endpoint, seven pts (58.3%) achieved CR, four pts (33.3%) had PR, one pt (9.1%) experienced progressive disease (PD). Based on the analysis of different subtype, the ORR and CR was 100% (5/5) and 80% (4/5) in AITL, 100% (4/4) and 25% (1/4) in PTCL-NOS. One ALK+ALCL and one PTCL-TFH patient achieved CR, while one SPTCL patient experienced progressive disease. Among the 12 pts who received the initial dose of 60mg, two pts did not complete the third course of treatment due to myelosuppression, and two pts reduced the dose to 40mg due to adverse reactions. Five pts in the 40mg group did not require dose reduction or withdrawal. The ORR was 87.5% (7/8) and the CR rate was 62.5% (5/8) in the 60 mg group. The ORR was 100% (4/4) and the CR rate was 50% (2/4) in the 40 mg group. Pts in the 40mg group had better tolerance and similar efficacy compared to those in the 60mg group. The most common (≥10%) Grade 3 or 4 treatment-related adverse events (AEs) were neutropenia (29%), thrombocytopenia (24%), and anemia (18%). No fatal AEs were reported at the end of follow-up, the median PFS and OS were not reached.
The integration of Selinexor into the standard tri-weekly CHOP chemotherapy regime has shown a manageable safety profile along with encouraging anti-tumor activity in patients with previously untreated PTCL. The trial is currently ongoing, and this manuscript presents the preliminary findings.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Selinexor (KPT-330) is a small molecule, oral, first-in-class, potent selective inhibitor of nuclear export (SINE) compound. Selinexor has received Food and Drug Administration approval for relapsed or refractory multiple myeloma and diffuse large B-cell lymphoma, and has shown significant anticancer activity across a range of preclinical models of cancer, including T-cell acute lymphoblastic leukemia. The high response rates seen in a phase ÃÆ'Ã'¢... clinical trial may warrant further investigation of the role of selinexor combined with chemical therapy in patients with PTCL.